![]()
| Nature 418, 929 - 930 (29 August 2002); doi:10.1038/418929a |
|
ALCINO J. SILVA AND SHEENA A. JOSSELYN
e-mail: silvaa@mednet.ucla.edu
Not everything that we learn is useful, so the brain needs a mechanism to prevent itself being burdened by unhelpful details. The molecular details of this mechanism are now being uncovered.
Studies of the molecular and cellular foundations of cognitive processes have come of age with the development of techniques that allow genes to be over-expressed, deleted or modified in mice. These altered animals have been studied from a variety of aspects simultaneously by molecular biologists, neurophysiologists and psychologists. The result is the birth of a field that is unravelling the basis of learning, remembering1, and now — as a paper in this issue shows — forgetting. On page 970, Genoux and colleagues2 report that an enzyme known as protein phosphatase 1 (PP1) actively suppresses memories in mice, both during and after a learning exercise. Like other biological processes, memory is regulated by yin-and-yang-like interactions between molecules with opposing functions — in this case, protein phosphatases and kinases, which respectively remove and add phosphate groups on target proteins, thereby altering their properties. To examine the role of these interactions in forming and maintaining memories, Genoux et al.2 generated mice that express a natural inhibitor of PP1, called inhibitor 1. When phosphorylated, this protein binds to PP1 and prevents it from working. The authors regulated the expression of inhibitor 1 in mice by using the reverse tetracycline transactivator system3: simply feeding the mice a tetracycline-like compound switches on the gene encoding the inhibitor, enabling PP1 activity to be blocked at will. To test the animals' memory, the authors trained them in an object-recognition task that takes advantage of the natural propensity of mice and other rodents to investigate new objects more avidly than familiar ones. Their memory for objects depends on the hippocampus — a brain structure that shows robust expression of the inhibitor. The authors' initial results help to explain one of the earliest discoveries of modern experimental psychology. In landmark experiments published in 1885, Hermann Ebbinghaus4 showed that distributing training into several sessions results in stronger memories than equivalent amounts of training crammed into a single session (students take note!). Genoux et al. now find that massed training triggers more PP1 activity than distributed training. Remarkably, when the authors switched on inhibitor 1 specifically during massed training, blocking the PP1 activity, this type of training became as effective as distributed training. So PP1 apparently suppresses memory formation during massed training — but how? One of PP1's targets is a gene-transcription factor called CREB, which becomes inactive when dephosphorylated by PP1. When phosphorylated, CREB directs the transcription of genes with specific CREB-binding DNA sequences in their control regions5, and so is needed for proteins to be produced from these genes. Since the late 1960s, it has been known that blocking protein synthesis results in memory deficits that emerge within hours of training6. Later studies of species ranging from the marine mollusc Aplysia to flies, mice and rats have put this finding on a more detailed molecular footing, showing that CREB is required for the long-term processes involved in memory formation7. It now appears that one of the ways in which inhibitor 1 enhances memory during massed training is by blocking the PP1-dependent dephosphorylation of CREB that would normally occur (Fig. 1). That alters CREB's activity, which Genoux et al. studied by looking at the CREB-regulated expression of an inserted gene encoding 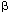 -galactosidase — an enzyme that produces a characteristic blue colour from its substrate. CREB activity (as judged by
-galactosidase — an enzyme that produces a characteristic blue colour from its substrate. CREB activity (as judged by 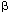 -galactosidase expression) was higher after distributed training than massed training when inhibitor 1 was not switched on. But when activated, the inhibitor abolished this difference, increasing CREB function during massed training to levels comparable to those during distributed training.
-galactosidase expression) was higher after distributed training than massed training when inhibitor 1 was not switched on. But when activated, the inhibitor abolished this difference, increasing CREB function during massed training to levels comparable to those during distributed training.
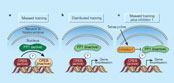
Figure 1
Learning and forgetting — a tale of molecular antagonism.
Full legend
High resolution image and legend (43k)
Earlier work8-10 showed that genetic manipulation of CREB also affects the learning regimen required to form long-term memories. In general, decreasing CREB levels increases the numbers of trials and the length of inter-trial intervals required for such memories to be formed, whereas increasing CREB levels has the opposite effect. Genoux et al.'s results suggest that the balance between the levels of active CREB and PP1 during training determines whether long-term memories are laid down.
The authors also found that inhibiting PP1 had almost immediate effects on memory formation. But these effects are unlikely to involve CREB-dependent transcription, which is an inherently slow process. Instead, these results point to molecules implicated in the early stages of memory formation, such as calcium/ calmodulin-dependent protein kinase II (CaMKII)11. During learning, this enzyme becomes activated by increases in calcium levels inside neurons, with the result that several critical signalling molecules at neuronal junctions (synapses) are phosphorylated, and CaMKII itself becomes constitutively active. Such events are thought to be central to the changes in neuronal communication required for learning and memory. Indeed, genetic and pharmacological manipulations that interfere with CaMKII impair learning11. Genoux et al. now show that inhibiting PP1 results in higher levels of activated CaMKII. So, during massed training PP1 suppresses both short-term and long-term processes involved in learning and memory formation.
Can this phosphatase also suppress memories after they have formed? Learning is thought to trigger molecular changes (short- and long-term) that facilitate neuronal communication and are important for memory. Just as digital information is encoded by altering the grooves in a compact disc during burning, so memories are encoded by altering synapses in the brain during training. By means not yet understood, neurons can decode these changes and use them to reconstruct the learned information. Unlike a compact disc, however, the brain seems to store information in a highly dynamic manner: psychological studies suggest that the brain cares more about the usefulness of stored memories than their fidelity. Most of what we perceive and process is quickly discarded, and what we do remember tends to fade and change with time. There is a good reason for this: flawless recall would burden the brain with useless details, perhaps at the expense of storing and recalling information that is useful.
Genoux and colleagues have found that PP1 is involved in forgetting. The authors trained mice to find a submerged platform in a round pool of opaque water — another task that requires the hippocampus. To test the animals' memory, Genoux et al. then removed the platform. The mice initially searched for the platform in the correct quadrant; but with time, memory faded and performance declined. The authors found that blocking PP1 activity during training accelerated learning, but did not affect memory decline. But impairing PP1 activity after learning all but halted the memory decline for four to six weeks. Interestingly, studies12 of an inserted CaMKII gene showed that interfering with its function after training destabilizes memory. So the antagonistic interactions between PP1 and CaMKII that are seen during learning may also be important later on, in maintaining memories.
Previous neurophysiological studies have shown that PP1, CaMKII and CREB modulate the changes in synaptic strength that are thought to underlie learning and memory13. These results mirror Genoux et al.'s molecular and behavioural findings. Similar examples of convergence between different types of study are now common in work on cognition at the molecular and cellular levels, showing that we are well on our way to unravelling the biology of learning and forgetting.
| 1. | Silva, A. J., Smith, A. M. & Giese, K. P. Annu. Rev. Genet. 31, 527-546 (1997). | Article | PubMed | ISI | ChemPort | |
| 2. | Genoux, D. et al. Nature 418, 970-975 (2002). | Article | PubMed | ISI | ChemPort | |
| 3. | Mayford, M., Mansuy, I. M., Muller, R. U. & Kandel, E. R. Curr. Biol. 7, R580-R589 (1997). | PubMed | ISI | ChemPort | |
| 4. | Ebbinghaus, H. Über das Gedachtnis. Untersuchungen zur Experimentellen Psychologie (Duncker & Humbolt, Leipzig, 1885). |
| 5. | Mayr, B. & Montminy, M. Nature Rev. Mol. Cell Biol. 2, 599-609 (2001). | Article | PubMed | ISI | ChemPort | |
| 6. | Davis, H. P. & Squire, L. R. Psychol. Bull. 96, 518-559 (1984). | Article | PubMed | ISI | ChemPort | |
| 7. | Silva, A. J., Kogan, J. H., Frankland, P. W. & Kida, S. in Annual Review of Neuroscience Vol. 21 (ed. Cowan, W. M.) 127-148 (Annual Reviews, Palo Alto, California, 1998). | ChemPort | |
| 8. | Yin, J., Del Vecchio, M., Zhou, H. & Tully, T. Cell 81, 107-115 (1995). | PubMed | ISI | ChemPort | |
| 9. | Josselyn, S. A. et al. J. Neurosci. 21, 2404-2412 (2001). | PubMed | ISI | ChemPort | |
| 10. | Kogan, J. H. et al. Curr. Biol. 7, 1-11 (1997). | PubMed | ISI | ChemPort | |
| 11. | Lisman, J., Schulman, H. & Cline, H. Nature Rev. Neurosci. 3, 175-190 (2002). | Article | PubMed | ISI | ChemPort | |
| 12. | Mayford, M. et al. Science 274, 1678-1683 (1996). | Article | PubMed | ISI | ChemPort | |
| 13. | Blitzer, R. D. et al. Science 280, 1940-1942 (1998). | Article | PubMed | ISI | ChemPort | |